
THE SCIENCE OF PRECIOUS PINK DIAMONDS: PLASTIC DEFORMATION
15.02.17
Last year, the ‘Unique Pink’ 15.38 carat pear shaped fancy vivid pink diamond was the most expensive pink diamond sold at auction in Geneva for $31.6m to an Asian buyer over the phone! Ever wondered what makes pink coloured diamonds so extraordinary and priceless? The IGR team are sharing some knowledge, below we tell you about the secrets and science behind pretty pink diamonds!
Pink diamonds are one of the most rare coloured diamonds and red are even more rare!
Colourless, yellow and blue diamonds have trace elements trapped, which generate colour. Whilst pink, red and brown diamonds obtain their colour from the geological phenomena of plastic deformation. What is plastic deformation? Plastic deformation is a natural geological process which has taken place for millions of years and shaped our world! The term sounds very complex but this post promises to keep it very simple and next time you are appreciating your favourite pink diamonds and hear about a priceless pink being sold, you will know the concept, science and geology behind those rosy hues!
In general, plastic deformation occurs because of tensile stress in the earth’s crust which encourages the dislocation and deformation of a material to change an object’s shape and size without breaking. Contrary to elastic deformation, plastic deformation is permanent.
The process of plastic deformation provides the source of colour in pink, red and brown diamonds by creating defects in the diamond’s atomic structure by rearranging the tightly arranged carbon atoms, this therefore alters the way in which light is absorbed and reflected by the diamond.
For a diamond, plastic deformation takes place under the earth’s surface during the post growth stage of the diamond’s crystal lattice. Diamonds grow at great depths and high temperatures in the earth, however the conditions in which plastic deformation takes place is unique. At these extreme temperatures, the diamond atoms become mobile and during the process of deformation the atoms dislocate and slip, especially along the glide plane in the directions parallel to the face of the octahedron. The stress and high pressures in the environment create ‘sheer’ force which pushes the atoms in the crystal in specific directions, initiating the dislocation of atoms or slipping crystal planes. The build up of the sheer force compresses and squeezes the shape whilst the crystal stays in once piece. Surprisingly, the individual carbon atoms separate from their neighbouring cells and reattach to the next atom along, which creates optical defects in the crystal lattice. These irregularities change the way the electrons absorb light; therefore, they are responsible for creation of precious pinks, rare reds and charming brown coloured diamonds.
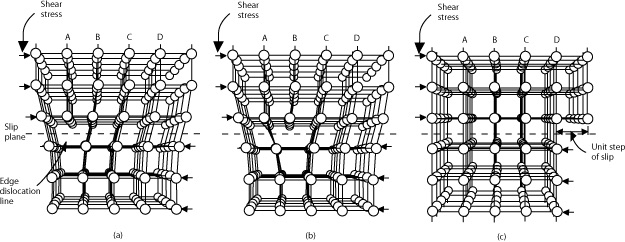
Diagram from https://www.nde-ed.org
The diagram demonstrates how the carbon atoms in a diamond move during the process of plastic deformation.
To delve further to the phenomena of plastic deformation there are two specific geological behaviours that take place to transform the diamond’s atomic structure: slipping and twinning. They both produce different result in terms of how the crystal structure generates colour. Studies and evidence in diamond growth demonstrates twinning occurs at high pressures of 900 °C, whilst the dislocation of atoms by slipping begins at much more extreme temperatures of 1300 °C.


Surface and internal graining were common features in the pink diamonds. Surface graining appears as a line or lines crossing facet junctions; Internal graining can appear in a number of different forms: as internal reflective planes as parallel whitish bands, and as an overall whitish haze. Photomicrographs by Vincent J. Cracco.
What is slipping and twinning?
The ‘sheer’ stress and force in the diamond’s environment cause the diamond’s atomic layers to slip over each other, like a pack of cards. Within the crystal plane, the atoms slip to fill in dislocations in the crystal layer. The action of slipping happens between the different layers and the slipping planes are where the atoms are most accumulated. The outcome of slipping is most evident in brown diamonds.
Twinning is a customary process in diamond growth, however in the development of plastic deformation, the deformed part of the crystal twins or duplicates the undistorted section. The result of mechanical twinning is most visible typically in natural pink purple diamonds. From a Siberian kimberlite diamond study, evidence shows these diamonds have mirror like reflections produced from the deformed lamellae/atomic boundaries found in the majority of pink diamonds.
Here’s a little more insight into the geology of how the diamond reaches the earth’s surface after the harsh journey it undergoes. The process of plastic deformation takes place during the destruction of deep seated rocks and in the long-distance transportation by kimberlite or lamproite magma to the surface of the earth, for example in case of the Daivik Mine, Canada. Explosive kimberlite magma eruptions rise through the keel zone bringing diamonds to the earth’s crust. Brown diamonds are blasted to the surface shortly after plastic deformation because the extreme environmental conditions would cause the body colour to fade if the diamond stayed longer in the earth.
We are done with the geology and science of plastic deformation. What is the result and what does this mean practically in terms of colour?
The outcome is diagnostic visual features such as strain patterns, vacancy clusters and graining within a diamond. These characteristics modify the body colour of the diamond and additionally are useful tools to identify a natural diamond.
The geological phenomena can result in strain patterns, that can be identified under the polariscope. Strain patterns are an advantageous indicator to demonstrate the transportation and growth from the internal stresses in the diamond’s atomic structure.
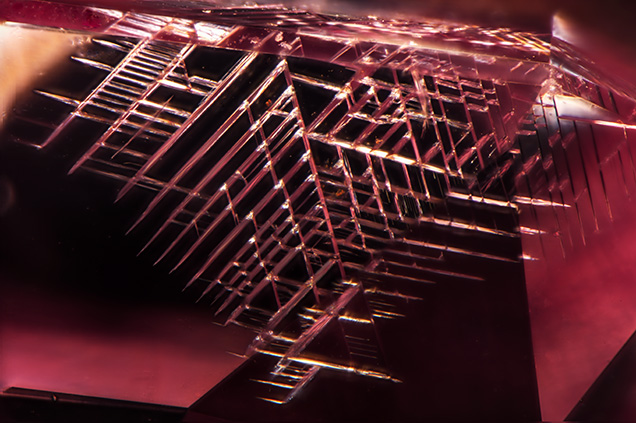
Photo by Jonathan Muyal
The photo shows strain patterns in a pink diamond, created from plastic deformation along the octahedral planes.

In the 0.59ct purple-pink diamond, the strong linear banded strain patterns observed with
polarised light polarised respond directly to the colour zoning and graining evident with darkfield illumination.
Vacancy clusters are group of vacancies that are defects (of an ambiguous structure) in the diamond’s atomic crystal. These clusters enhance diamond to absorption towards the blue end of the spectrum and is apparent in brown diamonds.
Another development of plastic deformation is ‘graining’ that is layers and areas of colour within a diamond crystal, usually interlaced with colourless zones therefore colour can be evenly or irregularly distributed. Graining is visible in both pink and brown diamonds and can be spotted under 10X magnification. Surface or lamellae graining is a very distinguishing feature of pink diamonds along planes of stress.

Uneven colour distribution in many “pink” diamonds, especially those that are type I. Bands are oriented along the octahedral planes; they probably are the result of plastic deformation of the diamond during its geologic history in the earth. Photomicrograph by John I.Koivula; magnified 15×.
The irregularities of straining, vacancy clusters and graining within the diamond lattice structure establishes colour characteristics that each have an exclusive absorption spectrum specifically for these diamonds. The lightest spectrum is pink, following into red and the darkest is brown.
Plastically deformed brown diamonds absorb light at the blue spectrum, more specifically in an absorption line of 503 nm and 494 nm. Brown diamonds from the Diavik mine were tested and ‘vacancy clusters’ are responsible for the brown hues and were increasing to shorter wavelengths and 500nm band absorptions.
The absorption band for pink plastically deformed diamonds is broad with an absorption of 560nm and collectively increasing absorption at shorter wavelengths. To be more specific, pink/purple Argyle diamonds absorption peaks are at 503,494 and 415nm.
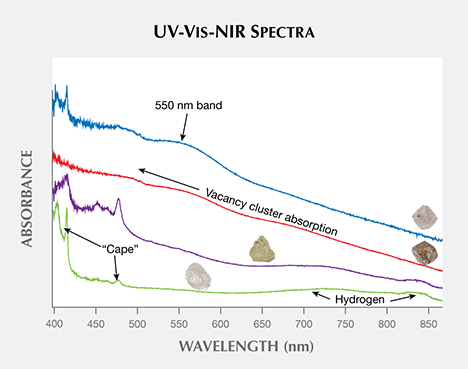
From Mining Diamonds in the Canadian Arctic: The Diavik Mine Article
Chart shows UV-Vis-NIR absorption spectra affecting the Diavik diamonds.
The source of colour for brown diamonds is solely plastic deformation therefore the diamond lacks any impurities, whilst for pink and red diamonds it’s a combination of plastic deformation and impurities, the exact recipe is still to be understood.
The journey of plastic deformation demonstrates the amazing phenomena that creates pink, red and brown diamonds so extraordinary! Even though the result is an imperfection in a diamond’s atomic structure, pink and red diamonds are rare and very sought after. I hope the next time you see a beautiful pink, red or even brown diamonds, you appreciate the amazing and treacherous journey these diamonds undergo and understand their high worth!
Dipika @ IGR London




